It all started with the fact that my camera point-and-shoot device flatly refused to work with batteries freshly removed from the charger - four AA-size NiMH batteries. Take them as usual and throw them away. But for some reason, this time curiosity prevailed over common sense (or maybe it was the toad that spoke), and I wanted to understand whether it was possible to squeeze at least something else out of these batteries. The camera is very hungry for energy, but there are also more modest consumers - wireless mice or keyboards, for example.
Actually, there are two parameters that are interesting to the consumer - the battery capacity and its internal resistance. There are also few possible manipulations - discharge and charge. By measuring the current and time during the discharge process, you can estimate the battery capacity. By the difference in battery voltage at idle and under load, you can estimate the internal resistance. By repeating the discharge-charge cycle (i.e., performing the “training”) several times, you can understand whether this action makes sense at all.
Accordingly, the following plan was formed - we make a controlled spark gap and charger with the ability to continuously measure process parameters, perform simple arithmetic operations on the measured values, and repeat the process the required number of times. We compare, draw conclusions, and finally throw away the batteries.
Measuring stand
A complete collection of bicycles. It consists of an analog part (in the diagram below) and a microcontroller. In my case, the intellectual part was the Arduino, although this is not at all important - as long as there is the necessary set of inputs/outputs.
The stand was made from what was found within a radius of three meters. If someone wants to repeat it, it is not at all necessary to follow the diagram exactly. The choice of element parameters can be quite wide, I will comment on this a little later.
The discharge unit is a controlled current stabilizer based on op-amp IC1B (LM324N) and field-effect transistor Q1. Almost any transistor, as long as there are enough permissible voltages, currents and power dissipation. And they are all small here. Feedback resistor and at the same time part of the load (together with Q1 and R20) for the battery - R1. Its maximum value must be such as to provide the required maximum discharge current. If we assume that the battery can be discharged to 1 V, then to ensure a discharge current of, for example, 500 mA, resistor R1 should not be more than 2 Ohms. The stabilizer is controlled by a three-bit resistive DAC (R12-R17). Here the calculation is as follows - the voltage at the direct input of the op-amp is equal to the voltage at R1 (which is proportional to the discharge current). We change the voltage at the direct input - the discharge current changes. To scale the DAC output to the desired range, there is a trimming resistor R3. It is better if it is multi-turn. The values of R12-R17 can be any (in the region of tens of kilo-ohms), the main thing is that the ratio of their values is 1/2. No special accuracy is required from the DAC, since the discharge current (voltage on R1) is measured directly by the instrumentation amplifier IC1D during the process. Its gain is K=R11/R10=R9/R8. The output is fed to the microcontroller ADC (A1). By changing the values of R8-R11, the gain can be adjusted to the desired value. The voltage on the battery is measured by the second amplifier IC1C, K=R5/R4=R7/R6. Why control the discharge current? The point here is basically this. If you discharge with a constant high current, then, due to the high internal resistance of worn-out batteries, the minimum permissible voltage of 1 V (and there is no other reference point for stopping the discharge) will be reached before the battery actually discharges. If you discharge with a constant low current, the process will take too long. Therefore, the discharge is carried out in stages. Eight steps seemed enough to me. If the hunt is more/less, then you can change the bit depth of the DAC. In addition, by turning the load on and off, you can estimate the internal resistance of the battery. I think that the controller operation algorithm during discharge does not require further explanation. At the end of the process, Q1 is locked, the battery is completely disconnected from the load, and the controller turns on the charge unit.
Charge block. Also a current stabilizer, only uncontrolled, but switchable. The current is set by the reference voltage source on IC2 (2.5 V, accuracy 1% according to the datasheet) and resistor R21. In my case, the charge current was classic - 1/10 of the nominal battery capacity. Feedback resistor - R20. You can use any other reference voltage source - depending on your taste and availability of parts. Transistor Q2 operates in a more rigid mode than Q1. Due to the noticeable difference between Vcc and battery voltage, significant power is dissipated across it. This is the price to pay for the simplicity of the circuit. But the radiator saves the situation. Transistor Q3 serves to force Q2 to turn off, i.e., to turn off the charge unit. Controlled by signal 12 of the microcontroller. Another reference voltage source (IC3) is needed for the controller’s ADC to operate. The measurement accuracy of our stand depends on its parameters. LED1 - to indicate the process status. In my case, it does not light up during the discharge process, lights up when charging and flashes when the cycle is completed.
The supply voltage is selected to ensure that the transistors open and operate in the required ranges. In this case, both transistors have a quite high gate unlocking voltage - about 2-4 V. In addition, Q2 is “backed up” by the battery voltage and R20, so the gate unlocking voltage starts from approximately 3.5-5.5 V. In turn The LM323 cannot raise the output voltage above Vcc minus 1.5 V. Therefore, Vcc must be quite large and in my case it is 9 V.
The charge control algorithm was based on the classic version of monitoring the moment the battery voltage begins to drop. However, in reality everything turned out to be not entirely true, but more on that later.
All measured values during the “research” process were written to a file, then calculations were made and graphs were drawn.
I think that everything is clear with the measuring stand, so let’s move on to the results.
Measurement results
So, we have charged (but non-functional) batteries, which we discharge and measure the stored capacity, and at the same time the internal resistance. It looks something like this.
Graphs on the axes: time, hours (X) and power, W (Y) for the best and worst batteries. It can be seen that the stored energy (the area under the graphs) is significantly different. In numerical terms, the measured battery capacities were 1196, 739, 1237 and 1007 mAh. Not a lot, considering that the nominal capacity (which is indicated on the case) is 2700 mAh. And the spread is quite large. What about internal resistance? It was 0.39, 0.43, 0.32 and 0.64 Ohm, respectively. Terrible. It is clear why the soap dish refused to work - the batteries are simply not able to deliver a large current. Well, let's start training.
Cycle one. Again the output power of the best and worst battery.

Progress is visible to the naked eye! The numbers confirm this: 1715, 1444, 1762 and 1634 mAh. The internal resistance also improved, but very unevenly - 0.23, 0.40, 0.1, 0.43 Ohm. It would seem there is a chance. But alas, further discharge/charge cycles did not produce anything. The capacitance values, as well as the internal resistance, varied from cycle to cycle within about 10%. Which lies somewhere close to the limits of measurement accuracy. Those. Long training, at least for my batteries, did nothing. But it became clear that the batteries retained more than half of their capacity and would still work at low current. At least some savings on the farm.
Now I want to dwell a little on the charging process. Perhaps my observations will be useful to someone who is planning to design a smart charger.
Here is a typical charge graph (on the left is the battery voltage scale in volts).

After the start of charging, a voltage dip is observed. In different cycles it may be greater or lesser in depth, of slightly different duration, and sometimes absent. Then, for about 10 hours, there is a uniform increase and then an almost horizontal plateau. The theory states that with a low charge current there is no voltage drop at the end of the charge. I was patient and still waited for this fall. It’s small (it’s almost invisible to the eye on the chart), you have to wait a very long time for it, but it’s always there. After ten hours of charging and before the decline, the voltage on the battery, although it increases, is extremely insignificant. This has almost no effect on the final charge; no unpleasant phenomena such as heating of the battery are observed. Thus, when designing low-current chargers, there is no point in equipping them with intelligence. A timer for 10-12 hours is enough, and no special accuracy is required.
However, this idyll was disrupted by one of the elements. After about 5-6 hours of charging, very noticeable voltage fluctuations occurred.

At first I attributed this to a design flaw in my stand. The photo shows that everything was assembled using a hinged installation, and the controller was connected with rather long wires. However, repeated experiments have shown that such nonsense consistently occurs with the same battery and never occurs with others. To my shame, I did not find the reason for this behavior. Nevertheless (and this is clearly visible on the graph) the average voltage value is growing as it should.
Epilogue
As a result, we have four batteries, for which an ecological niche has been found using precise scientific methods. We are disappointed in the capabilities of the training process. And we have one unexplained effect that occurs during charging.Next up is a larger battery - a car battery. But there the load resistors are a couple of orders of magnitude more powerful. Somewhere they are traveling across the expanses of Eurasia.
That's all. Thank you for your attention.
Federal Agency for Education
State educational institution of higher professional education
"TOMSK POLYTECHNIC UNIVERSITY"
Electrotechnical Institute
Direction 551300 – Electrical engineering, electromechanics and electrical technology
Department – Electric drive and electrical equipment
Abstract on the discipline
“Sources of guaranteed and uninterrupted power supply for industrial enterprises”
on the topic of NICKEL-METAL HYDRIDE BATTERIES
Students of group 7M142
Krupina N.V._______________
Kondrashov S.A. ____________
«_____»________________
Head Professor, Doctor of Technical Sciences
Garganeev A.G._______________
"_____"___________2009
Tomsk – 2009
Introduction
1. Terminology
3. Nickel-metal hydride batteries
4. Basic processes of Ni-MH batteries
5. Design of electrodes of Ni-MH batteries
6. Ni-MH battery design
7. Characteristics of Ni-MH batteries
8. Charging Ni-MH battery
9. Advantages and disadvantages of Ni-MH batteries
10. Standards and designations of NM batteries
11. Storage and operation of Ni-MH batteries
12. Manufacturers and prospects of NM batteries
13. Disposal
Conclusion
List of sources used
Introduction
It is almost impossible to imagine the modern world without any kind of electronic technology. Digital technologies have fit into our lives so well, making it more convenient and interesting, that we simply cannot refuse them.
However, we should not forget that for the operation of mobile devices, portable power supplies are needed that could meet the ever-increasing needs of modern electronics. We've gained WiFi and Bluetooth, freeing ourselves from data cables, but we still remain tied to electrical grids.
Applied science, however, does not stand still, offering more and more new types of electricity sources. On the other hand, it’s still strange that despite the presence of so many new technologies, the batteries of our phones, smartphones, PDAs and other gadgets are still dying. This happens because people think about proper handling of the battery only when it has completely failed and can be scrapped with peace of mind. It should be understood that replacing a battery can cost a pretty penny. We don’t argue that few people like to strictly follow the operating rules, but, unfortunately, only in this way can the battery life be maximized.
Today, batteries of five different electrochemical schemes are common: nickel-cadmium (Ni-Cd), nickel-metal hydride (Ni-MH), lead-acid (Sealed Lead Acid, SLA), lithium-ion (Li-Ion) and lithium-polymer (Li-Polymer). The determining factor for all of the listed batteries is not only portability (i.e., small volume and weight), but also high reliability, as well as long operating time. The main parameters of a battery are energy density (or specific energy by mass), number of charge/discharge cycles, charging and self-discharge rates. A lead-acid battery usually consists of two plates (electrodes) placed in an electrolyte (an aqueous solution of sulfuric acid). A nickel-cadmium cell has negative and positive plates rolled together and placed in a metal cylinder. The positive plate is made of nickel hydroxide and the negative plate is made of cadmium hydroxide. The two plates are insulated by a separator, which is moistened with electrolyte.
A nickel-metal hydride battery is structurally similar to a nickel-cadmium battery, but has a different chemical composition of the electrolyte and electrodes. In a lithium-ion battery, the electrodes and separator are placed in a lithium salt electrolyte.
There are a huge number of myths and legends about the supposedly ideal mode of operation, methods of “training”, storage, methods and modes of charging and restoring batteries, but let’s try to figure it out.
1.Terminology
A battery (from Latin accumulator - collector, accumulo - collecting, accumulating) is a device for storing energy for the purpose of its subsequent use. An electric battery converts electrical energy into chemical energy and provides the reverse conversion as needed. The battery is charged by passing electric current through it. As a result of the chemical reactions caused, one of the electrodes acquires a positive charge, and the other - a negative one.
A battery, as an electrical device, is characterized by the following basic parameters: electrochemical system, voltage, electrical capacity, internal resistance, self-discharge current and service life.
Battery capacity is the amount of energy that a fully charged battery should have. In practical calculations, capacity is usually expressed in ampere-hours (
). The number of amp hours indicates the period of time that a given battery will operate at 1 amp of current. It is worth adding, however, that modern mobile devices use much lower currents, so battery capacity is often measured in milliamp-hours (or, or mAh). The nominal capacity (as it should be) is always indicated on the battery itself or on its packaging. However, the actual capacity does not always coincide with the nominal capacity. In practice, the actual battery capacity ranges from 80% to 110% of the nominal value.Specific capacity is the ratio of the battery capacity to its dimensions or weight.
A cycle is one sequence of charging and discharging a battery.
Memory effect is the loss of battery capacity during its operation. It manifests itself in the tendency of the battery to adapt to the duty cycle in which the battery has been operating for a certain period of time. In other words, if you charge a battery several times without completely discharging it first, it seems to “remember” its state and next time simply will not be able to discharge completely, therefore, its capacity decreases. As the number of charge-discharge cycles increases, the memory effect becomes more pronounced.
Under such operating conditions, an increase in crystals on the plate occurs inside the battery (the structure of batteries will be discussed below), which reduce the surface of the electrode. With small crystalline formations of the internal working substance, the surface area of the crystals is maximum, therefore, the amount of energy stored by the battery is also maximum. When crystalline formations become larger during operation, the surface area of the electrode decreases and, as a result, the actual capacity decreases.
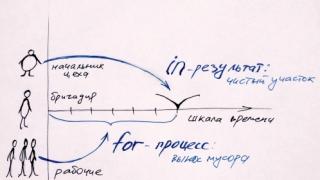
Figure 1 shows the effect of the memory effect.

Figure 1 – Memory effect.
Self-discharge is the spontaneous loss of stored energy by a battery over time. This phenomenon is caused by redox processes that occur spontaneously, and is inherent in all types of batteries, regardless of their electrochemical system. To quantify self-discharge, the amount of energy lost by the battery over a certain time is used, expressed as a percentage of the value obtained immediately after charging. Self-discharge is maximum in the first 24 hours after charging, so it is estimated both for the first day and for the first month after charging. The amount of battery self-discharge depends largely on the ambient temperature. Thus, when the temperature rises above 100°C, self-discharge can double.
2. Batteries: types and origin
The leading positions in the battery production market are occupied by Japan, Taiwan, China, and South Korea, and they are constantly increasing the scale of their “modest” presence in the world market.
There are dozens of different battery designs on the market today, and each manufacturer is trying to achieve the optimal combination of characteristics - high capacity, small size and weight, performance in a wide temperature range and in extreme conditions.
At the same time, studies show that more than 65% of mobile and portable technology users want even more capacious batteries, and they are willing to pay a lot of money for the ability to use their “car” (or phone) for several days without recharging. That is why, in most cases, it is necessary to purchase a more capacious battery than the one included in the kit.
According to the electrochemical system, batteries are divided into several types:
Lead-acid (Sealed Lead Acid, SLA);
Nickel-cadmium (Ni-Cd);
Nickel metal hydride (Ni-MH);
Lithium-ion (Li-Ion);
Lithium polymer (Li-Pol);
Fuel.
Lead-acid batteries are no longer used in modern portable electronics, so we will begin our excursion with nickel batteries, still used in batteries for cameras, laptops, video cameras and other devices.
The ancestor of nickel batteries were nickel-cadmium (Ni-Cd) batteries, invented back in 1899 by the Swedish scientist Waldmar Jungner. The principle of their operation was that nickel acts as a positive electrode (cathode), and cadmium acts as a negative electrode (anode). At first, it was an open battery, in which the oxygen released during charging went straight into the atmosphere, which prevented the creation of a sealed case and, coupled with the high cost of the necessary materials, significantly slowed down the start of mass production.

How should a Ni─MH battery be restored and why is it important?
Ni─MH batteries are advertised by manufacturers as batteries with high energy capacity, resistant to cold, and free from the disadvantages of cadmium batteries. Indeed, this type of battery does not contain such a harmful substance as cadmium. The production and processing of Ni─MH batteries does not have the same difficulties as for Ni─Cd. But they still have some disadvantages of cadmium batteries. For example, the “memory effect” remained. And in general, Ni─MH are very sensitive to charging and discharging modes. Nickel-metal hydride batteries require advanced devices to charge. In addition, in order to extend the service life of such elements, they need to be periodically restored. Let's talk about how this can be done.
Despite the advantages of nickel-metal hydride batteries over nickel-cadmium batteries, they have a number of disadvantages. And they must be taken into account during operation.

To begin with, it should be noted that Ni─Cd is more expensive. True, technology does not stand still and the price of these types of batteries is gradually becoming comparable. In this case, we are talking about batteries of the common form factor AA (“finger”) and AAA (“little finger”). have a more pronounced “memory effect”, but, nevertheless, nickel-metal hydride batteries also face this problem.
Nickel metal hydride batteries have fewer charge-discharge cycles. The first deterioration in their performance characteristics is observed after 200-300 charge-discharge cycles. This type of battery has a higher self-discharge compared to Ni─Cd batteries (about 1.5 times).
One more point is worth noting. Nickel-metal hydride batteries can deliver high current, but it is not recommended to set values greater than 0.5*C when discharging. This leads to a significant reduction in the number of charge-discharge cycles and a decrease in service life. For now, where high discharge currents are required, Ni─Cd batteries are still used.
Do not forget that a charger for Ni-MH batteries will work without problems with nickel-cadmium batteries, but not vice versa.
Charging nickel-metal hydride batteries
Charging of nickel-metal hydride batteries can be drip and fast. Trickle charging is not recommended by manufacturers due to the difficulty in detecting when current flow to the battery has stopped. As a result, severe overcharging and battery degradation may occur. As a rule, Ni─MH batteries are charged using a fast or accelerated charging option. At the same time, the charging efficiency is higher than with drip charging. The charge current in this case is set to 0.5─1C.

Due to the “memory effect”, nickel metal hydride cells can lose a significant part of their capacity. It manifests itself less than in nickel-cadmium, but is still present. The memory effect manifests itself during repeated cycles of incomplete discharge and subsequent charging. As a result of such operation, the battery “remembers” an increasingly lower discharge limit, which is why the capacity decreases. Part of the active mass of the battery falls out of the process.

To eliminate this effect, it is recommended to regularly recondition or train the batteries. To do this, a charger or light bulb discharges the battery to 0.8-1 volts, and then completes the charging process. If the battery has not been restored for a long time, it is recommended to do several such cycles. The recommended frequency of such training is once a month.
Manufacturers of Ni─MH batteries claim that the “memory effect” takes about 5 percent of the capacity. Restoring this amount of capacity as a result of training is quite possible. In principle, this can be measured by discharging a fully charged battery. To do this, you will need to detect the discharge time and multiply it by the discharge current. This will be the capacity that needs to be compared with the nominal value. Some devices, for example, take measurements automatically.
An important point when restoring Ni─MH batteries is that the charger has a battery discharge function with minimum voltage control. This is necessary in order to prevent the battery from being deeply discharged during recovery (below 0.8-1 volts). This is indispensable for those cases when you do not know the initial state of charge of the battery, and it is not possible to estimate the approximate discharge time.
When you do not know the state of charge of the battery, you need to discharge it with a light bulb or other resistance under constant voltage control. Otherwise, such restoration of the battery will end in its deep discharge. If you are restoring an entire battery with elements connected in series, it is better to first fully charge them to equalize the degree of charge.
In general, regarding the restoration of nickel-metal hydride batteries, the following point should be noted. If the battery has already worked for several years, then such restoration by completely discharging and charging may be useless. Such restoration is useful as periodic preventive maintenance during battery operation. The fact is that during the operation of Ni─MH batteries, in parallel with the occurrence of the “memory effect,” a change in the composition and volume of the electrolyte occurs. For nickel-cadmium batteries, there are examples of restoration by adding distilled water to the cells. This was discussed in the article about.
I would also like to note that it is best to restore the elements separately, and not the entire battery.
Among other batteries, NiMH batteries are often used. These batteries have high technical characteristics that allow you to use them as efficiently as possible. This type of battery is used almost everywhere; below we will look at all the features of such batteries, and also analyze the nuances of operation and well-known manufacturers.
Contents
What is a Nickel Metal Hydride Battery
To begin with, it is worth noting that nickel-metal hydride is a secondary power source. It does not produce energy and requires recharging before use.
It consists of two components:
- anode – nickel-lithium or nickel-lanthanum hydride;
- cathode – nickel oxide.
An electrolyte is also used to excite the system. Potassium hydroxide is considered the optimal electrolyte. This is an alkaline food source according to modern classification.
This type of battery replaced nickel-cadmium batteries. The developers managed to minimize the disadvantages characteristic of earlier types of batteries. The first industrial designs were put on the market in the late 80s.
At the moment, it has been possible to significantly increase the density of stored energy in comparison with the first prototypes. Some experts believe that the density limit has not yet been reached.
Operating principle and design of Ni-Mh battery
First, it’s worth considering how a NiMh battery works. As already mentioned, this battery consists of several components. Let's look at them in more detail.
The anode here is a hydrogen-absorbing composition. It is capable of absorbing large amounts of hydrogen; on average, the amount of absorbed element can exceed the volume of the electrode by 1000 times. To achieve complete stabilization, lithium or lanthanum is added to the alloy.
Cathodes are made from nickel oxide. This allows you to obtain a high-quality charge between the cathode and anode. In practice, a variety of cathode types can be used depending on their technical design:
- lamella;
- metal-ceramic;
- metal felt;
- pressed;
- nickel foam (polymer foam).
Polymer foam and metal felt cathodes have the highest capacity and service life.
The conductor between them is alkali. Concentrated potassium hydroxide is used here.
The design of the battery may vary depending on the purposes and tasks. Most often, these are an anode and a cathode rolled into a roll, between which there is a separator. There are also options where the plates are placed alternately, arranged with a separator. A mandatory design element is a safety valve; it is activated when the pressure inside the battery emergency rises to 2-4 MPa.
What types of Ni-Mh batteries are there and their technical characteristics
All Ni-Mh batteries are Rechargeable Battery (translated as rechargeable battery). Batteries of this type are produced in different types and shapes. All of them are intended for a variety of purposes and tasks.
There are batteries that are almost never used at the moment, or are used to a limited extent. Such batteries include the “Krona” type, it was marked 6KR61, they used to be used everywhere, now they can only be found in old equipment. Batteries of type 6KR61 had a voltage of 9v.
We will analyze the main types of batteries and their characteristics that are used now.
- AA.. The capacity ranges from 1700-2900 mAh.
- AAA.. Sometimes labeled MN2400 or MX2400. Capacity – 800-1000 mAh.
- WITH. Medium-sized batteries. They have a capacity in the range of 4500-6000 mAh.
- D. The most powerful type of battery. Capacity from 9000 to 11500 mAh.
All listed batteries have a voltage of 1.5v. There are also some models with a voltage of 1.2v. Maximum voltage 12v (by connecting 10 1.2v batteries).
Pros and cons of Ni-Mh battery
As already mentioned, this type of battery replaced older varieties. Unlike analogues, the “memory effect” was significantly reduced. We also reduced the amount of substances harmful to nature used during the creation process.
 Battery pack of 8 1.2v batteries
Battery pack of 8 1.2v batteries The advantages include the following nuances.
- Work well at low temperatures. This is especially important for equipment used outdoors.
- Reduced “memory effect”. But still he is present.
- Non-toxic batteries.
- Higher capacity compared to analogues.
Batteries of this type also have disadvantages.
- Higher self-discharge value.
- More expensive to produce.
- After approximately 250-300 charge/discharge cycles, the capacity begins to decrease.
- Limited service life.
Where are nickel metal hydride batteries used?
Due to their large capacity, such batteries can be used everywhere. Whether it is a screwdriver or a complex measuring device, in any case, such a battery will provide it with the required amount of energy without any problems.
In everyday life, such batteries are most often used in portable lighting devices and radio equipment. Here they show good performance, maintaining optimal consumer properties for a long time. Moreover, both disposable elements and reusable ones, regularly recharged from external power sources, can be used.
Another application is instruments. Due to their sufficient capacity, they can also be used in portable medical equipment. They work well in blood pressure monitors and glucometers. Since there are no voltage surges, there is no influence on the measurement result.
Many measuring instruments in technology have to be used outdoors, including in winter. Here metal hydride batteries are simply irreplaceable. Due to their low response to negative temperatures, they can be used in the most difficult conditions.
Operating rules
It must be taken into account that new batteries have a fairly high internal resistance. To achieve some reduction in this parameter, you should discharge the battery to zero several times at the beginning of use. To do this, you should use chargers with this function.
Attention! This does not apply to disposable batteries.
You can often hear the question to how many volts can you discharge a Ni-Mh battery. In fact, it can be discharged to almost zero parameters, in which case the voltage will not be enough to maintain the operation of the connected device. It is even recommended to sometimes wait until the battery is completely discharged. This helps reduce the “memory effect”. The battery life is accordingly extended.
Otherwise, the operation of batteries of this type does not differ from analogues.
Is it necessary to pump Ni-Mh batteries?
An important stage of operation is pumping up the battery. Nickel metal hydride batteries also require this procedure. This is especially important after long-term storage in order to restore capacity and maximum voltage.
To do this, you need to discharge the battery to zero. Please note that electric shock is required. As a result, you should get the minimum voltage. This way you can revive the battery, even if quite a lot of time has passed since the date of manufacture. The longer the battery has been sitting, the more charging cycles are required. Typically, it takes 2-5 cycles to restore capacitance and resistance.
How to restore NiMH battery
Despite all the advantages and features, such batteries still have a “memory effect”. If the battery begins to lose performance, then it should be restored.
Before starting work, you need to check the battery capacity. Sometimes it turns out that it is almost impossible to improve the performance, in which case you just need to replace the battery. We also check the battery for malfunction.
The work itself is similar to pumping. But here they do not achieve a complete discharge, but simply reduce the voltage to a level of 1v. It takes 2-3 cycles. If during this time it was not possible to achieve the optimal result, the battery should be considered unusable. When charging, you need to maintain the Delta Peak parameter for a specific battery.
Storage and disposal
It is worth storing the battery at a temperature close to 0°C. This is the optimal state. It is also necessary to take into account that storage should occur only during the expiration date; this data is indicated on the packaging, but the decoding may differ from one manufacturer to another.
Manufacturers worth paying attention to
All battery manufacturers produce Ni-Mh batteries. In the list below you can see the most famous companies offering similar products.
- Energizer;
- Varta;
- Duracell;
- Minamoto;
- Eneloop;
- Camelion;
- Panasonic;
- Irobot;
- Sanyo.
If you look at the quality, they are all about the same. But we can highlight Varta and Panasonic batteries; they have the most optimal price-quality ratio. Otherwise, you can use any of the listed batteries without any restrictions.
The main difference between Ni-Cd batteries and Ni-Mh batteries is the composition. The base of the battery is the same - it is nickel, it is the cathode, but the anodes are different. For a Ni-Cd battery, the anode is cadmium metal; for a Ni-Mh battery, the anode is a hydrogen metal hydride electrode.
Each type of battery has its pros and cons, knowing them you can more accurately select the battery you need.
| pros | Minuses | |
| Ni-Cd |
|
|
| Ni-Mh |
|
|
Will the old charger fit the new battery if I change the Ni-Cd to a Ni-Mh battery or vice versa?
The charging principle for both batteries is absolutely the same, so the charger can be used from the previous battery. The basic rule for charging these batteries is that they can only be charged after they are completely discharged. This requirement is a consequence of the fact that both types of batteries are subject to the “memory effect”, although with Ni-Mh batteries this problem is minimized.
How to properly store Ni-Cd and Ni-Mh batteries?
The best place to store a battery is in a cool, dry room, since the higher the storage temperature, the faster the battery self-discharges. The battery can be stored in any condition other than completely discharged or fully charged. The optimal charge is 40-60%%. Once every 2-3 months, you should recharge (due to the presence of self-discharge), discharge and charge again to 40-60% of the capacity. Storage for up to five years is acceptable. After storage, the battery should be discharged, charged and then used normally.
Can I use batteries with a larger or smaller capacity than the battery from the original kit?
Battery capacity is the operating time of your power tool on battery power. Accordingly, there is absolutely no difference in battery capacity for a power tool. The actual difference will only be in the charging time of the battery and the operating time of the power tool from the battery. When choosing a battery capacity, you should proceed from your requirements; if you need to work longer using one battery, choose more capacious batteries; if the included batteries are completely satisfactory, then you should choose batteries of equal or similar capacity.